

Dave Humphreys
2027 Skoda Epiq review: Quick drive
2 Hours Ago
Air pollution is a serious health hazard, and catalytic converters, SCR and EGR are all used to minimise this.

Contributor


Contributor
Pure fossil fuels are made up of hydrocarbons and this means that when they’re burned using oxygen from the air, they should produce no emissions apart from carbon dioxide and water.
In reality, modern petrol and diesel fuels available from the typical service station contain hundreds of additives and additional chemicals. These additions cause the fuels to burn less cleanly, resulting in the emission of gases such as nitrogen oxide (commonly known as NOx), carbon monoxide, nitric oxide and other volatile organic compounds (VOCs) such as acetone and benzene.
Many of the gases and compounds described above are very hazardous to our health by contributing to air pollution. Beyond particulate filters, carmakers have implemented technologies to reduce the emission of these gases into the atmosphere.
Three of these technologies which are commonplace in the automotive industry include catalytic converters, selective catalytic reduction (SCR) and exhaust gas recirculation (EGR).
MORE: Particulate filters: What are they?
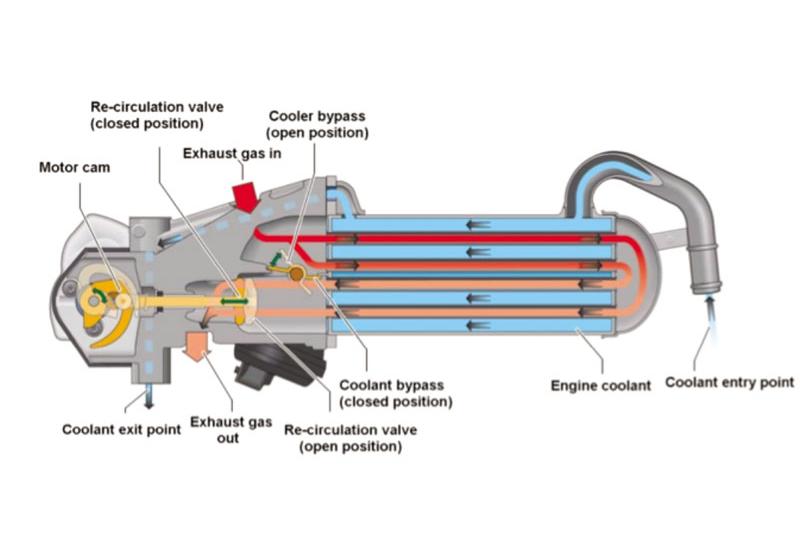
Before emissions from an engine are treated through catalytic processes or particulate filters, EGR acts as an initial step used by carmakers to reduce harmful pollutants.
At a basic level, the primary part of an EGR system is the EGR valve. This valve is located after the combustion chamber (engine cylinders), and can open to different levels, as controlled by the engine control unit (ECU). Through this, the valve precisely regulates the amount of exhaust gas that is recirculated and mixed with fresh intake air.
Post-combustion exhaust gas is largely devoid of oxygen. Therefore, as the proportion of exhaust gas that is recirculated back into the combustion chamber increases, the amount of oxygen that enters correspondingly reduces, lowering the temperature of the combustion chamber. A cooler combustion chamber consequently results in a reduction in the amount of nitrogen oxides and carbon dioxide produced.
Today, many EGR systems bolster this with an EGR cooler to further reduce peak combustion temperatures. Nevertheless, for situations where a high combustion temperature is desirable, such as during particulate filter regeneration or to warm up the catalytic converter or SCR process, a bypass flap is used to prevent the cooler from lowering exhaust gas temperature.

Conceptually, catalytic converters have been around since the end of the 19th century, but first gained widespread use in cars during the 1970s in North America following the introduction of more stringent emissions regulations by the US Environmental Protection Agency (EPA).
Engineer Eugene Houdry is credited with designing the first modern catalytic converter for automotive purposes, receiving a patent for the design of a generic version in 1956.
With the introduction of unleaded fuel to Australia in 1986, all petrol vehicles are required to have a catalytic converter, which forms an integral part of a car’s exhaust system.
A catalytic converter is an ovoid tube-shaped device with a stainless steel body, with an inlet to receive raw gases and emissions from the engine, and an outlet that allows these gases to flow through to the tailpipe or particulate filter (if equipped).

The steel body contains the key part that enables the catalyst to function, namely a ceramic honeycomb. Many catalytic converters may also incorporate oxygen sensors (also known as lambda sensors) that are linked to the car’s ECU.
The term ‘catalytic’ in ‘catalytic converter’ provides an important clue as to how the device operates – specifically, by employing substances that work as catalysts and react with gases coming from the engine through the inlet port.
The ceramic honeycomb is coated with these catalyst materials, which consist of rare metals including platinum, rhodium and palladium, with a total quantity of 5-7g used. These metals are highly valuable, and their presence is one reason why catalytic converters are susceptible to theft.
These metals trigger a redox (reduction-oxidation) reaction with the gases passing through the honeycomb. The reduction catalyst, usually a rhodium-platinum oxide, reduces nitrogen oxides (NOx) into their harmless nitrogen and oxygen component gases. The oxidation catalyst, usually also made from platinum or palladium, adds oxygen to carbon monoxide to form carbon dioxide.
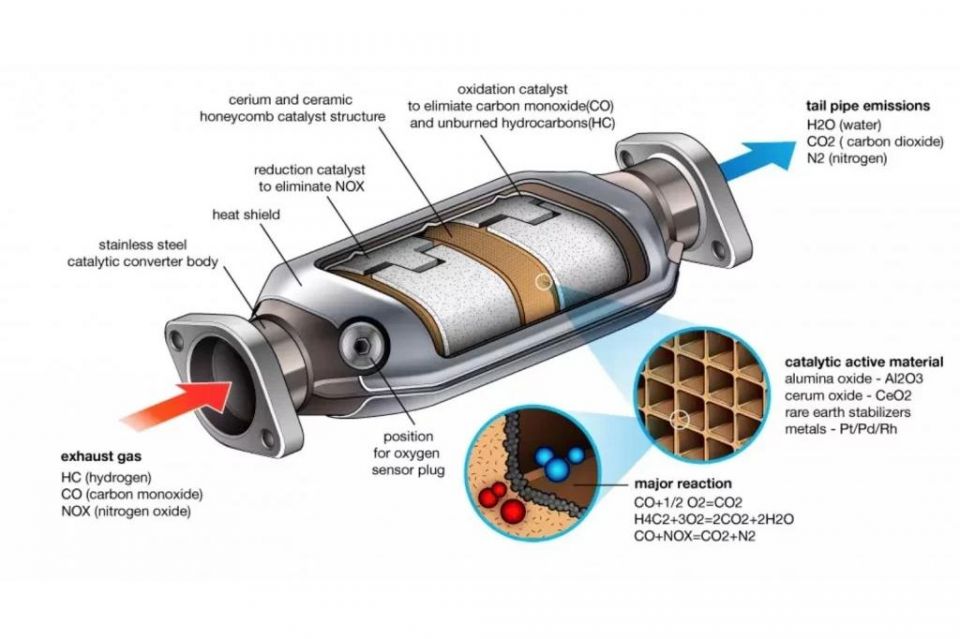
Another oxidation reaction also takes care of any unburnt fuel or other hydrocarbons, by also converting them into carbon dioxide and water.
These three reactions form the basis of the industry standard ‘three-way’ catalytic converter. The oxygen sensor is used to improve the efficiency of these reactions by allowing the ECU to determine whether the air to fuel mixture should be altered to enable a greater amount of oxygen to flow into the catalytic converter.
Note that catalytic converters need an internal temperature of at least 300°C for the redox reactions to occur, which often caused early converters to not function properly until the car’s engine was warmed up.
Catalytic converters also generally don’t work well with (now phased out) leaded fuel, as lead can build up on the honeycomb and compromise the converter’s operation.
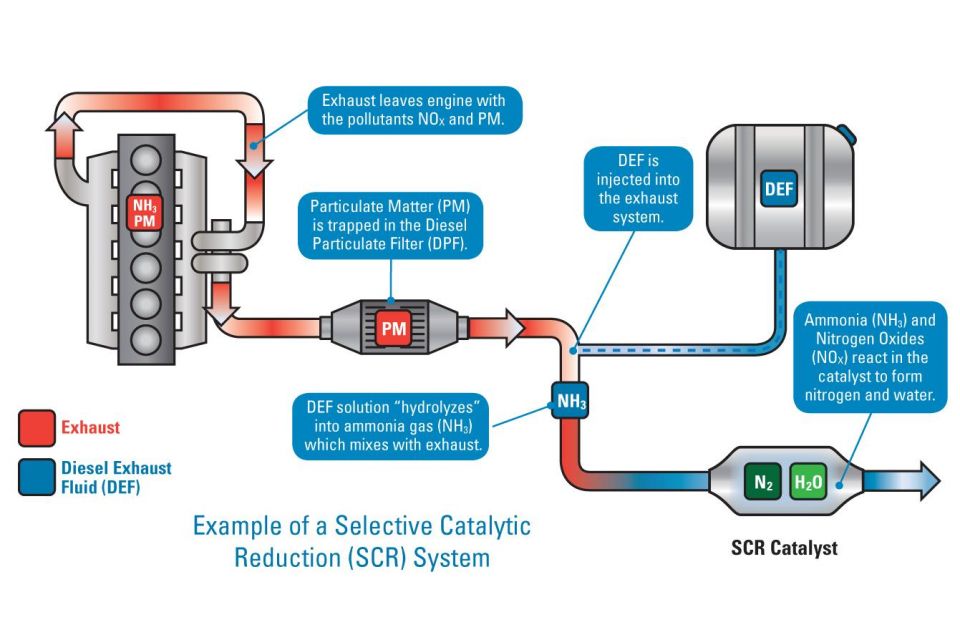
Diesel engines are known to produce large quantities of nitrogen oxides, and tightening emissions regulations mean that the typical three-way catalytic converter may not be effective enough to remove these to the standard required.
SCR is a more advanced emissions control technology that is used with diesel engines. In a vehicle equipped with SCR, the oxidation and reduction reactions formerly taking place within a single catalytic converter are split across two distinct processes.
The first of these is the oxidising catalyst process, which remains in place and undertakes the same reactions (as described above) to remove carbon monoxides and other hydrocarbons.
Secondly, SCR is then used as part of a separate reduction reaction process that selectively targets nitrogen oxides, hence its name.
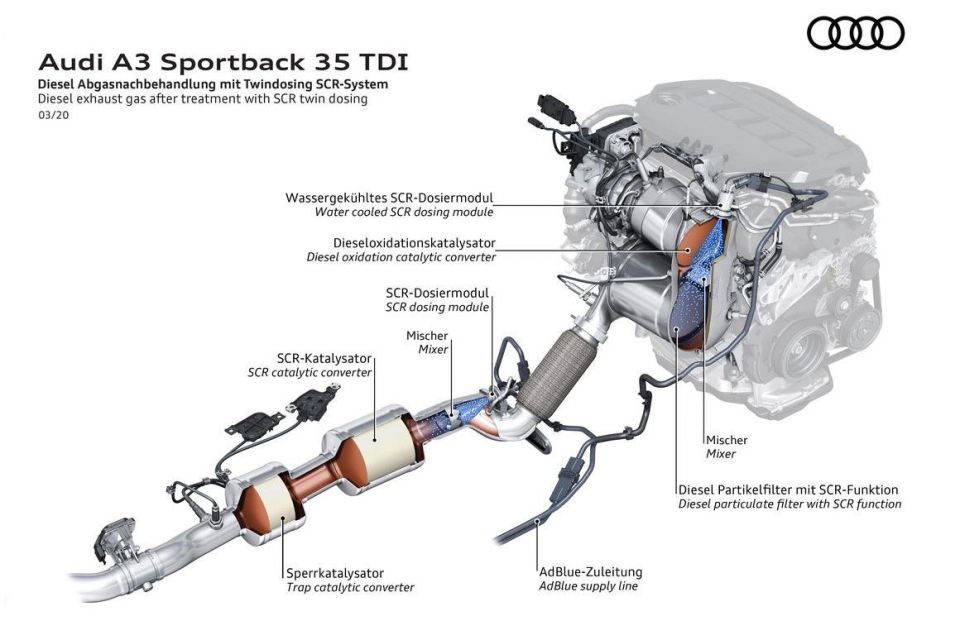
The SCR process itself consists of two key components, namely a liquid reducing agent, known as a DEF (Diesel Exhaust Fluid) that is injected as needed, and a housing directly connected to the car’s exhaust system that provides a setting for any subsequent reduction reactions to occur.
Ammonia would be an ideal chemical to use as a reducing agent to break down these nitrogen oxides, however in pure form it remains very toxic, and so a solution of urea in water is used instead. In the DEF context, this is often known by the brand name AdBlue, and consists of a solution of 32.5 per cent urea in demineralised water.
When this solution is injected into a car’s exhaust system, high temperatures facilitate its conversion to ammonia gas. The ammonia gas then acts as a catalyst to break down the nitrogen oxides into harmless nitrogen and water.
AdBlue itself has been in the news recently as Australian supplies of the fluid have dwindled. Some diesel engines may enter into a ‘limp mode’ or cease to function if not refilled with the substance.

MORE: What happens if I run out of AdBlue? MORE: More Australian-made AdBlue coming soon


Dave Humphreys
2 Hours Ago

Damion Smy
3 Hours Ago


William Stopford
4 Hours Ago

CarExpert
5 Hours Ago


William Stopford
5 Hours Ago


Damion Smy
6 Hours Ago
Add CarExpert as a Preferred Source on Google so your search results prioritise writing by actual experts, not AI.