

Ben Zachariah
2026 KGM Musso EV review
4 Hours Ago
Hydrogen is the most abundant resource. How can it be used as a fuel for vehicles?

Contributor


Contributor
With only one electron per atom, hydrogen is the lightest, simplest and most abundant element in existence. Compared to natural gas, it contains about three times as much energy for the same weight.
At normal air pressure, however, hydrogen has a very low energy content for its volume. This low density means it must also be pressurised to have any meaningful use as a fuel for vehicles.
In the automotive context, hydrogen cars – known as fuel cell electric vehicles (FCEVs) – run by converting hydrogen into electricity.
Perhaps the best way to understand how a fuel cell does this is to compare its operation to that of a battery, such as those found in standard battery electric vehicles (BEVs).
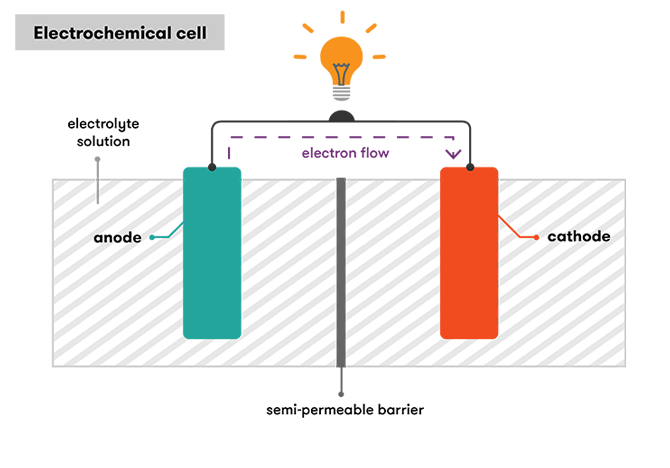
The fundamental principle behind a battery is the conversion of stored chemical energy to electricity. Through a chemical reaction known as a redox (reduction-oxidation) reaction one terminal of a battery, the anode, donates electrons to the cathode terminal or electron acceptor.
The anode is able to donate these electrons by being oxidised through its interaction with a material known as an electrolyte. By being sandwiched between these two terminals, the electrolyte also acts as an internal barrier to ensure electrons can only flow from anode to cathode (which is ‘reduced’ through the addition of electrons) when they are connected via an outer circuit, rather than directly within the battery.
In a BEV, the outer circuit passes through a motor, which uses the flow of electrons to drive the wheels.
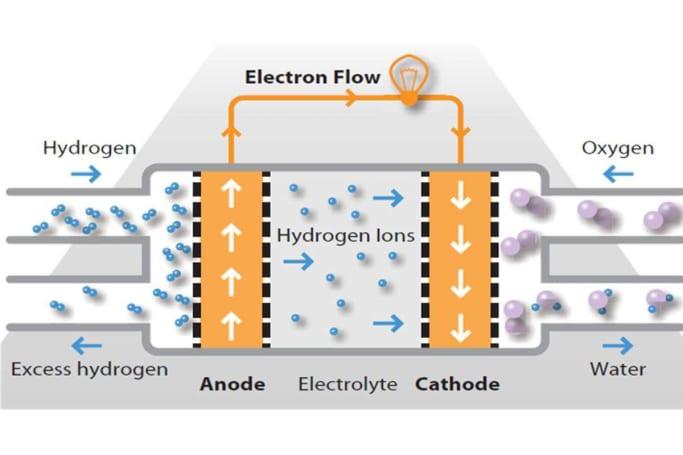
In a fuel-cell, the hydrogen gas acts as the electron donor instead of the chemical that constitutes the anode in a battery. A fuel cell still includes an anode, however instead of donating electrons it removes a hydrogen atom’s sole electron, leaving behind what is known as a positively charged, electron-less hydrogen ion.
The electrolyte continues to serve as a barrier, in this case allowing the hydrogen ions directly through to the cathode whilst forcing the separated electrons to flow through an outer circuit which incorporates an electric motor that drives the car.
The voyage of the electrons and the hydrogen ions, however, is not yet complete. As the electrons pass through the motor and the outer circuit, they meet up again at the cathode with their hydrogen ion friends, which were able to take the more direct route through the electrolyte.
In a fuel cell, the cathode consists of oxygen. This interacts with the now recombined, complete hydrogen atoms to form HO – water, which then leaves the fuel cell as its sole emission.
Apart from the environmental benefit of water being the only emission of FCEVs, their key advantages over a battery electric vehicle (BEV) stems from their refuelling time and range.
Unlike a typical BEV which, even with rapid DC charging, may take anywhere from 30 mins to hours to fully charge, the refuelling time for a hydrogen vehicle is closer to its petrol and diesel counterparts, taking around five minutes.
The other key benefit is range, with the latest hydrogen vehicles such as the Hyundai Nexo and Toyota Mirai both allowing customers to drive in excess of 600km before having to refuel, a handy upgrade over the 300-400km targeted by most BEVs today.
The primary drawback restricting more widespread adoption of hydrogen vehicles is the lack of refuelling infrastructure. Although more common in California and Japan, currently the only hydrogen refuelling station in Australia is at Hyundai headquarters in Macquarie Park, Sydney, along with a portable unit stored at Toyota in Melbourne.

Other drawbacks relate to the hydrogen vehicle itself. Hydrogen gas in ambient conditions has a very low density. Therefore, for an FCEV to have a useful range it must compress and store hydrogen within highly pressurised tanks, typically around 10,000 psi. This compares to around 3600psi for compressed natural gas and only 30-40psi for the air within your car’s tyres!
The space required for these hydrogen tanks, together with cooling requirements for the fuel-cell itself, creates challenges for vehicle engineering and design, and how best to manage the extra weight to maintain vehicle performance and handling. It’s unlikely there will be a sporty hydrogen car anytime soon.


Hydrogen burns when exposed to air, and this has also caused concerns with the perceived safety of FCEVs if the gas leaks during a crash. However, FCEVs implement a number of features such as reinforced tanks and an automatic hydrogen shut-off valve to mitigate these risks.
As of writing, there are no hydrogen vehicles in Australia available for sale to the general public. However, hydrogen vehicles available overseas include the Hyundai Nexo, Toyota Mirai, and the Honda Clarity.
In some cases, these vehicles are only available to lease with added incentives such as US$15,000 of bonus hydrogen refuelling.
MORE: Hydrogen Hyundai trucks in Australia by 2025

The Nexo mentioned above is the first hydrogen vehicle to receive ADR (Australian Design Rules) certification, meaning it can legally be driven on Australian roads. The ACT Government has recently ordered a fleet of 20 Nexos, and they may be available for sale to the general public in the near future.
Toyota Australia has been running a small fleet of Mirai fuel-cell vehicles as part of a trial.
Additionally, the Commonwealth Government has recently launched a $300 million Advancing Hydrogen Fund to fund better refuelling infrastructure, and construction of a refuelling station in Canberra is underway, alongside plans to build more stations in Melbourne and Brisbane.
Although OEMs such as Honda, Toyota and Hyundai are currently undertaking R&D into hydrogen cars, heavy haulage may be especially suited to the technology. Fleet operators that maintain a range of road trains or long haul trucks that operate point-to-point would not require a widespread refuelling network, and would be able to take full advantage of the longer range offered by hydrogen fuel cells.
Whilst fast-charging battery technology is rapidly improving, in truck applications, it would take significantly less time to refuel a large hydrogen tank compared to recharging an equivalent range battery.

MORE: Filling it up– E85 MORE: Filling it up – Biodiesel MORE: Filling it up – LPG MORE: Filling it up – CNG MORE: Fuel ratings explained


Ben Zachariah
4 Hours Ago


James Wong
4 Hours Ago


James Wong
4 Hours Ago


James Wong
4 Hours Ago


Damion Smy
11 Hours Ago


William Stopford
12 Hours Ago
Add CarExpert as a Preferred Source on Google so your search results prioritise writing by actual experts, not AI.